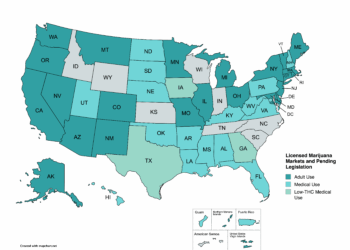
The clock has started ticking with the May 21 posting in the Federal Register to reclassify cannabis to Schedule III of the Controlled Substances Act.
Optimists believe that given President Joe Biden’s political motivation to get this done quickly, we may actually have a final rule before the election. The fact that Attorney General Merrick Garland signed and submitted the proposed rule himself, apparently without DEA leadership support, is a strong sign of that.
However, past scheduling efforts through what is known as a “formal rulemaking process” signal otherwise. Expected legal challenges in what has historically been a long process could drag this out until next year at the soonest, possibly years.
One research company chief executive, who recently won an indefinite postponement of a hearing on a proposed rule to classify a couple of psychedelic drugs to Schedule I, told CRB Monitor News he will be requesting a public hearing and possibly an injunction on the marijuana proceedings.
The formal rulemaking process
The publishing of the proposed rule kicks off a 60-day public comment period. Comments must be postmarked or submitted electronically by July 22.
Additionally, the public has a 30-day period to request a hearing in writing before an Administrative Law Judge (ALJ).
The CSA allows the attorney general to add to, transfer or remove drugs from any of the five classification schedules by rulemaking “made on the record after opportunity for a hearing” pursuant to the Administrative Procedure Act. Usually, the DEA will kick off this process as the attorney general’s agent. In this case, DEA Administrator Anne Milgram did not.
Many thought that the proposed rule would be subject to the Administration’s Office of Management and Budget for a review period of up to 90 days before posting on the Federal Register. But that’s not so with rules made under this process, according to Executive Order 12866, signed by President Bill Clinton in 1993. The DEA has made that clear in other proposed rules, most recently an April 11 proposal to permanently place some synthetic opioids on Schedule I.
However, the DOJ recognized taking cannabis to Schedule III would have “unique economic impacts” involving a multibillion-dollar industry.
“DOJ acknowledges that there may be large impacts related to Federal taxes and research and development investment for the pharmaceutical industry, among other things. DOJ is specifically soliciting comments on the economic impact of this proposed rule,” it said in the proposal.
If an ALJ hearing is requested, it’s up to the DEA administrator to grant the hearing, post another public notice in the Federal Register and assign the judge.
Although the Administration wants this done quickly, there appears to be no formal time constraints on the DEA regarding when the hearing must be held, said attorney Agustin Rodriguez, a partner at Troutman Pepper.
In a proposed rule filed in December 2023 to classify two psychedelic drugs to Schedule I, the DEA didn’t authorize an ALJ hearing until March 28, scheduled for June 10. But the hearing has been stayed while Panacea Plant Sciences challenges the proposed rule in U.S. District Court.
An ALJ hearing is held like a formal trial. The judge will allow time for parties to provide arguments and evidence.
“The ALJ will have all the powers necessary to conduct a fair hearing, to take all necessary action to avoid delay, and to maintain order,” the proposed rule says.
However, any ruling on the positions is just a recommendation, which the DEA administrator could accept or reject. And rejection has happened before.
In 1988, in a hearing for a petition by the advocacy group NORML to take the plant down to Schedule II, a DEA judge ruled marijuana “is one of the safest therapeutically active substances known to man” and should be available for medical use. But then-DEA Administrator John Lawn rejected that ruling, and his decision was sustained by a federal appellate court. Marijuana has remained in Schedule I ever since.
The DEA then considers all the evidence and writes a final rule. Once a final rule is published, it will include an effective date. Rodriguez said he’s seen effective dates as short as 30 days, but it could be 90 days.
This time, there is the added wrinkle that if cannabis is rescheduled, the DEA would have to develop additional rules to comply with international treaty obligations.
“Concurrent with this rulemaking, DEA will consider the marijuana-specific controls that would be necessary to comply with relevant treaty obligations in the event that, after the hearing, a final order reschedules marijuana, and, to the extent such controls are needed if marijuana is rescheduled, will seek to finalize any such regulations as soon as possible.”
Where there’s a will there’s a way
The Department of Justice announced the proposed rule May 16, just 19 months after Biden asked Garland and Secretary of Health and Human Services Xavier Becerra to reconsider marijuana’s placement as a Schedule I drug.
“The timing of this announcement indicates the Administration is committed to getting a final rule in place before the election,” Rodriguez said in an email that day.
During a webinar held by the U.S. Cannabis Council on May 22, three industry attorneys were optimistic that the political will of the Biden Administration could push this through quickly.
“It’s always important to look at what the DOJ needs to accomplish,” said attorney Kelly Fair, a partner at Dentons. “Sixty days is aggressive but reasonable.”
She said the DEA is already considering the Department of Health and Human Services’ August opinion to reschedule, as well as other research, and is considering what rules will be needed for international treaties.
She said a hearing could be held in August, but it will be a “wild card” as to how long the DEA will take to review all the comments and evidence.
Fair also said that the ALJ hearing is not a time to “filibuster” the proposed rule. There must be new evidence.
While the law allows “aggrieved” parties to challenge the DOJ action in U.S. District Court, their standing would have to be proven. She said it’s important to remember this is not a descheduling proposal, and anyone who objects on diversion or illicit market grounds will have a tough time saying they have standing.
Jesse Alderman, co-chair of the cannabis practice at Foley Hoag, said during the webinar that he thinks there will be a hearing in 90 days, possibly late summer or early fall.
He said one of the “foremost” grounds for legal attack is that the HHS adopted a different test to determine whether a drug has a currently acceptable medical use (CAMU) than what the DEA has been using for years. But, he noted that the Office of Legal Council’s opinion was “very, very strong.”
The OLC opinion sought by the attorney general said the DEA’s current approach was “impermissibly narrow, and that satisfying HHS’s two-part inquiry is sufficient to establish that a drug has a CAMU even if the drug has not been approved by FDA and would not satisfy DEA’s five-part test.”
Fair said we could get a final rule by the election, not considering legal challenges.
“We may see milestones before the election,” she said. “Will the process be completed? Probably not.”
A complicated process could lead to court challenges
David Heldreth, CEO of Panacea Plant Sciences, laughed out loud when told some attorneys believe this proposed rule could be finalized before the election.
“I can promise you, that ain’t happening,” he told CRB Monitor News.
Panacea Plant Sciences researches psychedelics and cannabis for medicinal use. Heldreth has also personally used cannabis medicinally for his own ailments. He fully supports descheduling, but he said he will be filing a request for an ALJ hearing on the rescheduling of marijuana.
Heldreth is not a lawyer, but he has been representing the Bellevue, Wash., company pro se. And he’s been through this scheduling process a couple times before.
In April, he filed a complaint in U.S. District Court for the Western District of Washington, challenging the DEA’s attempt to move 2,5-dimethoxy-4-iodoamphetamine (DOI) and 2,5-dimethoxy-4-chloroamphetamine (DOC) to Schedule I. In that case, Heldreth claims that scheduling these drugs would make them illegal and result in a government seizure of the product his company possesses.
Under constitutional law, he says, the taking of property by the government must be heard in U.S. District Court and not through the DEA’s ALJ procedure. On April 24, ALJ Paul Soeffing granted a stay of the DEA hearing until the District Court renders its verdict.
Noting that the government seemed to concede to a stay, Soeffing said in the order, “Although the public interest may lie in an expeditious and efficient resolution to these proceedings, the public interest also lies in ensuring parties are not potentially subjected to an adjudication process found unconstitutional.”
While Heldreth’s argument in that case would not be in play for rescheduling marijuana, he still plans to request an ALJ hearing and will challenge the procedure on other grounds.
He said he will ask for descheduling in his hearing request. “Our company does not have a DEA license for drugs in Schedules I, II or III. It gives me standing. It harms me while advancing another group, those with Schedule III licenses.”
He will also mention in his hearing request that cultivators with DEA licenses should be allowed to dispense the plant.
In his experience, it could be 60 days after the close of the public comment period before a hearing is announced. Counsel needs to be admitted, which the DEA can challenge. Then the judge requests comments and sets deadlines.
He said anything that deviates from the Administrative Procedure Act would allow a party to pull the case into District Court, like he is doing with the DOC and DOI scheduling.
Heldreth said he also plans to challenge the procedure in federal court because the DEA did not consult with tribal nations under Executive Order 13175.
“This rule does not have substantial direct effects on one or more Indian Tribes, on the relationship between the Federal Government and Indian Tribes, or on the distribution of power and responsibilities between the Federal Government and Indian Tribes,” the proposed rule states.
Heldreth, who says his family is Cherokee, disagrees. “I want them to follow and respect tribal nations.”
Finally, what the DEA, or Garland, does with the ALJ ruling could also lead to lawsuits.
“The ALJ decision is not binding at all until reviewed by the DEA, and they can say, ‘I disagree,’” Heldreth said. “But that gives a reason to sue.”
He thinks that’s potentially why Garland signed the proposed rule instead of Milgram. “They don’t want to give her that chance.”
Hilary Bricken, partner at Husch Blackwell law firm who has worked in the cannabis space for 14 years, agreed that a final rule by the end of the year is “never going to happen.” She said one drug took 14 years before there was a final rule.
“Procedure is a big one where these regulatory agencies get attacked,” she said in an interview.
Despite the president asking for a scheduling review, Bricken doesn’t believe this is a high priority of Biden’s. “I don’t think this administration cares about protecting state-legal cannabis businesses at all.”
Meanwhile, marijuana prohibitionists such as Smart Approaches to Marijuana (SAM) are gearing up to fight the scheduling change. SAM CEO Kevin A. Sabet sent an email on May 17 saying the proposal was anticipated, but the, “DEA’s argument is crafted in a way that indicates they see the rationale for rescheduling as largely lacking a strong basis.”
The organization is working with a team of lawyers specializing in administrative law to craft a strong challenge. “Far from losing hope, our sense is there is a real chance for victory,” Sabet said.